The Choi lab published a new article in Nucleic Acids Research, entitled “Binding of microRNA-122 to the hepatitis C virus 5’ untranslated region modifies interactions with poly(C) binding protein 3 and the NS5B viral polymerase” This work was in collaboration with Dr. Stan Lemon lab at UNC.
https://academic.oup.com/nar/advance-article/doi/10.1093/nar/gkad1000/7370024
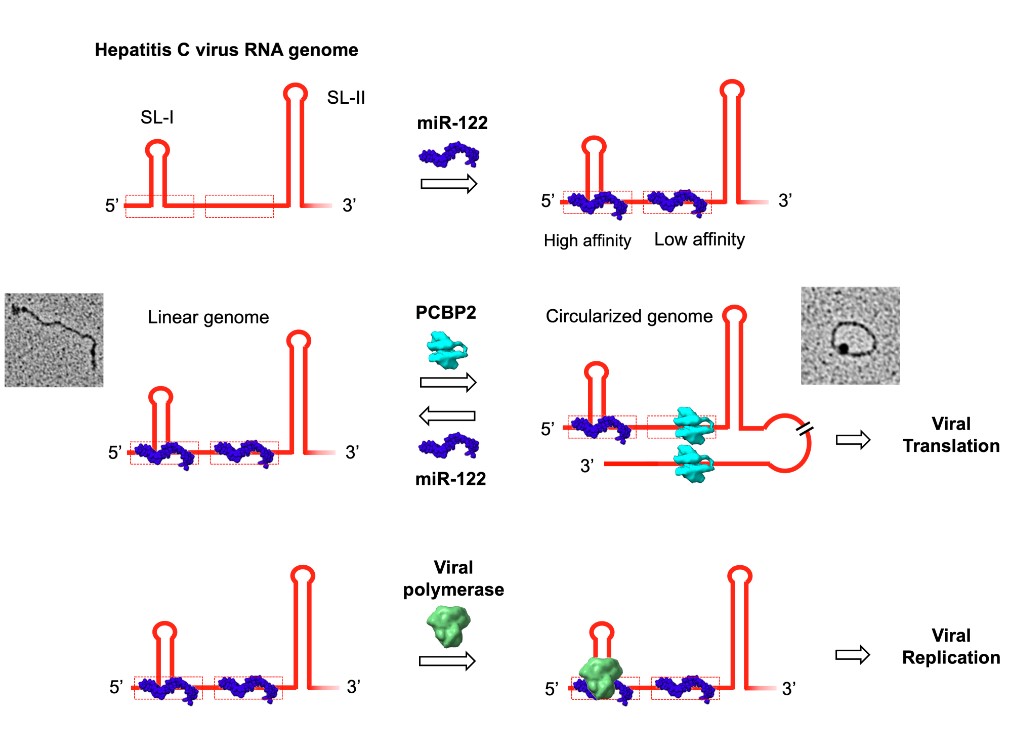
Abstract: Hepatitis C virus (HCV) requires two cellular factors, microRNA-122 (miR-122) and poly(C) binding protein 2 (PCBP2), for optimal replication. These host factors compete for binding to the 5′ end of the single-stranded RNA genome to regulate the viral replication cycle. To understand how they interact with the RNA, we measured binding affinities of both factors for an RNA probe representing the 5′ 45 nucleotides of the HCV genome (HCV45). Isothermal titration calorimetry revealed two, unequal miR-122 binding sites in HCV45, high-affinity (S1) and low-affinity (S2), differing roughly 100-fold in binding affinity. PCBP2 binds a site overlapping S2 with affinity similar to miR-122 binding to S2. PCBP2 circularizes the genome by also binding to the 3′ UTR, bridging the 5′ and 3′ ends of the genome. By competing with PCBP2 for binding at S2, miR-122 disrupts PCBP2-mediated genome circularization. We show that the viral RNA-dependent RNA polymerase, NS5B, also binds to HCV45, and that the binding affinity of NS5B is increased in the presence of miR-122, suggesting miR-122 promotes recruitment of the polymerase. We propose that competition between miR-122 and PCBP2 for HCV45 functions as a translation-to-replication switch, determining whether the RNA genome templates protein synthesis or RNA replication.

 The College of Arts
The College of Arts